Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Analysis of The Detection of Polymorphisms in The Elastic Fiber System Gene rs2018736 A456C Of the FBLN 5 Gene in Women with Post Hysterectomy Genital Prolapse
*Corresponding author: Athanasia Sergounioti, Department of Clinical Microbiology, General Hospital of Amfissa, Oikismos Drosohoriou, Amfissa 33100, Greece.
Received: July 14, 2022; Published: July 25, 2022
DOI: 10.34297/AJBSR.2022.16.002282
Abstract
The article presents the results of molecular genetic studies of the rs2018736 A456C gene of the FBLN5 gene in women with posthysterectomy genital prolapse. 92 women aged 31 to 67 were examined. The results of the study showed that the C allele and hetero/homozygous genotypes of the rs2018736 A456C polymorphism of the FBLN 5 gene are significant markers of an increased risk of PHEGP in women of the Uzbek population (P<0.004). (χ2=27.30; p<0.0006; OR=10.81; 95%CI 2.35-49.68). The A allele and the functionally favorable A/A genotype are reliable protective markers for the development of pathology (χ2=30.84; p<0.001; OR=6.37; 95%CI 3.21 -12.63).
Keywords: Posthysterectomy Genital Prolapse; Phegp; Genetics; A456C FBLN5 Gene
Introduction
Currently, in practical medicine, genital prolapse with occurrence varying from 1.4 to 48.9% [1-11] occupies one of the leading places in the structure of gynecological morbidity. Conducted studies have shown familial cases and racial differences in the prevalence of genital prolapse [10]. Atoyan MR, Makaeva ZZ describe the genetic determinism of genital prolapse. The most common pathological changes that require surgical correction after hysterectomy are vaginal wall prolapse and pelvic floor relaxation. Prolapse of the vaginal cuff after hysterectomy by laparotomy in women who have not had genital prolapse previously is 2 to 5%. The incidence of vaginal vault prolapse after hysterectomy is 0.2 to 43% [2,7,12-15]. It should be noted that in the structure of gynecological interventions, reconstructive plastic surgery takes the leading third place after benign tumors of the genitals and endometriosis. There are more than 300 methods of surgical treatment of this pathology by vaginal, abdominal, laparoscopic, or combined approaches. However, despite the variety of correction methods, there is still evidence of a high incidence of recurrence from 6 to 38.7% of cases, which causes dissatisfaction with the functional results of operations [14-15]. The condition of the connective tissue of various structures of the pelvic floor largely determines the pathogenesis of the development of pelvic organ prolapse. Mechametal showed that one of the key connective tissue proteins directly involved in the synthesis and integration of elastic fibers is the matrix protein fibulin-5 (FBLN-5). The adequacy of the elastic component of the connective tissue ensures its elasticity, extensibility, and flexibility, which is of great importance both during childbirth and the postpartum, recovery period [16]. With an increase in the number of hysterectomies around the world, including in Uzbekistan, the number of patients with Post-Hysterectomy Genital Prolapse (PHEGP) tends to increase, which requires close attention and comprehensive study [17].
Recently, in many gynecological diseases, great importance has been attached to the study of molecular genetic aspects of the development of pathology. Today, in the solution of the issues of the Pelvic Organs Pathology (POP), determining the initial signs of destruction of the Connective Tissue (CT), i.e., destruction of elastin as a marker of initial preclinical signs of the development of the pelvic organ’s pathology is of immediate interest. It is known that CT is formed from numerous cells and intercellular substance - proteoglycans and glycoproteins (i.e., adhesive proteins) and various collagen, elastic, and reticular fibers. It should be noted that numerous elastic fibers in the vaginal wall are conducive to maintaining elasticity, stretching under the influence of a certain force. The condition of elastic fibers - elastic chains are protected from the outside by glycoproteins, microfibrils, including fibrillins (genes FBN1, FBN3), as well as fibulin genes (FBLN1, FBLN2, FBLN15) [18]. Fibulins are heat shock proteins that mediate the connection between the elastin core and microfibrils, and the structure of elastic fibers [16]. In this regard, the study of the pathogenesis of the development of post-hysterectomy genital prolapses, considering the molecular genetic aspects of the development of pathology, will reveal new opportunities for primary prevention of morbidity [19-21]. The purpose of our study was to assess the detectability of polymorphisms of the elastic fiber system gene rs2018736 A456C of the FBLN gene for the risk of developing pathology of the pelvic organs, namely the development of post-hysterectomy genital prolapses.
Material and Methods of Research
We examined 55 women with Post-Hysterectomy Genital Prolapse (PHEGP) aged 30 to 67 years. All patients underwent clinical, instrumental, functional examinations. For molecular genetic testing, 4 ml whole blood was taken. Written informed consent was obtained from all patients. The control group consisted of 37 patients of the corresponding age. As part of the research, a genetic analysis of biological blood samples taken from 92 patients presented to determine the genotypic polymorphism of the FBLN 5 gene, consisting of the A456C alleles (rs2018736), was conducted. DNA/RNA was isolated from all biological blood samples using the Ribo-prep kit (Interlabservis, Russia). Allelespecific primers from the manufacturer were selected from DNA samples to identify the polymorphism of the genotype consisting of the A456C (rs2018736) alleles of the FBLN 5 gene. 92 DNA samples were studied for their genotyping by Polymerase Chain Reaction (PCR). For this, the Applied Biosystems Veriti 96-well fast thermal cycler was optimized according to the following program: initial denaturation was conducted once at 94°C for 180s, at 94°C for 10s, at 64°C for 10s, 72°C for 20s. To trigger the polymerase chain reaction, these steps were repeated 40 times. A statistical analysis of the results was carried out using the statistical software package “Open Epi 2009, Version 2.3”.
Research Results
Among the 55 examined patients with PHEGP, there were 9 women aged 31 to 40 years, 24 women aged 41 to 50 years, 12 women aged 51 to 60 years, and 10 women over 60 years of age. Whereas in the control group without PHEGP, there were 3 women aged 20 to 30 years, 4 women aged 31 to 40 years, 22 women aged 41 to 50 years, 5 women aged 51 to 60 years, and 3 patients over 60 years of age, respectively.
The results of molecular genetic testing of the A456C gene (rs2018736) of the FBLN5 gene showed that the functional allele A in the control group of women without PHEGP was found in 79.7% of cases (59/74), and in the main group of women with PHEGP, allele A was found in 38.2% of cases (42/110), which was 2.08 times lower than in the control group. (χ2=30.84; p<0.001; OR=0.16; 95%CI 0.08-0.31). Whereas the non-functional (mutant) allele “C” was detected in 68 cases - in the group of women with PHEGP, which amounted to 61.8% (68/110), while in the control group of subjects without PHEGP, the mutant allele “C” was detected in 15 cases (15/74), which amounted to 20.3%. (χ2=30.84; p<0.001; OR=6.37; 95%CI3.21 -12.63); [Table 1].
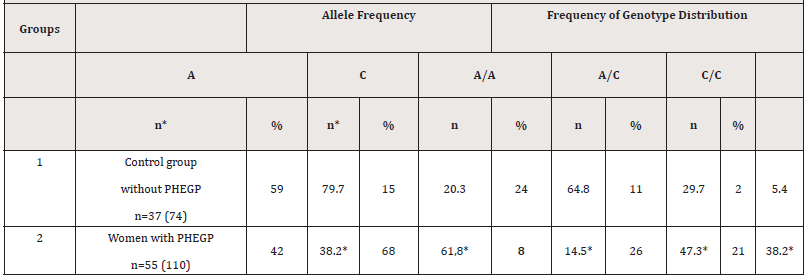
Table 1:The frequency of distribution of the rs2018736 A456C polymorphism genotypes of the FBLN 5 gene in the group with PHEGP and the control group.
The results of analyzing the frequency of alleles of the studied polymorphisms of the FBLN 5 gene among patients with PHEGP and in the control group of women show statistically significant difference. (P<0.05) The data obtained show that the obtained high value of the odds ratio (OR=6.37) indicates the dependence of the association between the mutant allele “C” of the polymorphism (rs2018736) of the FBLN 5 gene and post-hysterectomy genital prolapse [Table1]. Analysis of the association of polymorphisms of the FBLN 5 gene genotypes in the examined patients showed distinctive features [Table 1]. Thus, favorable A/A genotypes of the FBLN 5 gene in the control group of patients occurred in 64.8% (24/37) cases, while in the main group this genotype was determined in 14.5% (8/55) cases, which is 4.5 times lower compared to the control group (P<0.05). (χ2=27.30; p<0.0006; OR=0.09; 95% CI 0.03-0.25).
The heterozygous A/C genotype of the association of the rs2018736 A456C polymorphism of the FBLN 5 gene was found in the main group of patients with PHEGP in 26 patients, which accounted for 47.3% of cases. Whereas in patients of the control group, this genotype A/C was found in 11 women, which accounted for 29.7% of cases, respectively, which was 1.6 times lower compared to the active treatment group. (P<0.05) (χ2=27.30; p<0.0006; OR=2.12; 95% CI 0.88-5.12) [Table 2].
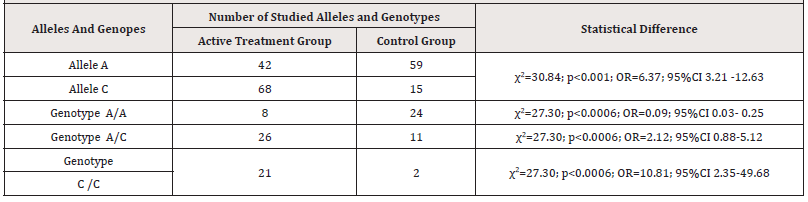
Table 2:The frequency of distribution of the rs2018736 A456C polymorphism genotypes of the FBLN 5 gene in the group with PHEGP and the control group.
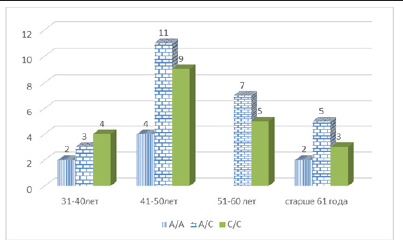
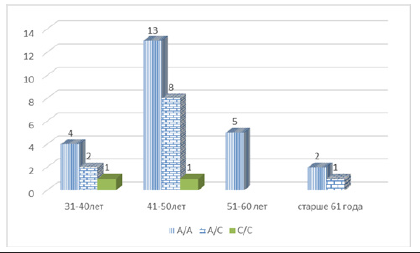
The unfavorable homozygous C/C variant rs2018736 A456C of the FBLN 5 gene was found in 21 patients in the active treatment group, which was 38.2% (21/55), and in the control group, this genotype was found in 2 out of 37 women, which was 5.4 % of cases (5/37), respectively. (χ2=27.30; p<0.0006; OR=10.81; 95%CI 2.35-49.68). Given that in the main group of women with PHEGP, the detectability of the unfavorable homozygous C/C variant of the FBLN 5 gene is high and exceeds the indicators of control subjects by 7.07 times, the data obtained may indicate that the carrier state of the homozygous genotype of the rs2018736 A456C polymorphism of the FBLN5 gene may be a factor of predisposition to the development of this pathology, increasing its risk by 7.1 times (OR=10.81)[Table 2]. We analyzed the detectability of the association of polymorphisms of the FBLN 5 gene genotypes, considering the age of patients in the active treatment group. As follows from the figure, the heterozygous A/C variant and the unfavorable C/C variant of genotypes were most often identified at the active working age of 41-50 years (11 and 9, respectively). However, the point of interest is the detectability of the unfavorable homozygous C/C variant of the FBLN 5 gene at the age of 31-40 years in 4 patients.
As follows from the figure, in the control group of patients without PHEGP, the heterozygous A/C variant and the unfavorable C/C homozygous variant of the FBLN 5 gene genotypes at the age of 31-40 years was detected in 3 patients and at the age of 41-50 years in 9 patients. The data obtained indicate that the unfavorable allelic C variant of the FBLN 5 gene is detected at almost all ages, however, it was most often found in the adult population over 50 years of age. The risk of PHEGP development is most often noted in the adult population; however, the observed rate detectability of the unfavorable allelic variant at a young, active age requires close attention from obstetrician-gynecologists. Thus, the results of molecular genetic testing have shown that a possible association of the unfavorable “C” variant allele of the rs2018736 A456C polymorphism of the FBLN 5 gene, leading to the replacement of A with C at position 456 of the amino acid sequence, with the development of post-hysterectomy genital prolapse in women. It was found that the risk of developing PHEGP in women in the presence of a variant polymorphism allele in the C genome increased by 6.4 times (OR=6.37). It should be noted that the heterozygous A/C genotype of the rs2018736 A456C polymorphism of the FBLN 5 gene is a genetic determinant that predisposes to the development of this pathology, which increases its risk by 2 times (OR=2.12). The data obtained requires close attention from obstetriciangynecologists.
According to the literature data, the population frequency of occurrence of various allelic variants and genotypes of polymorphic genes can be variable, as it is influenced by various dynamic factors involved in creating the genetic structure of the population. At the same time, the assessment of the expected and observed frequency of genotypes of the studied polymorphic genes potentially associated with the development and pathogenesis of diseases, which can be determined in accordance with the distribution of frequencies according to the Hardy-Weinberg (HW) equilibrium, is of great importance (Table 3).
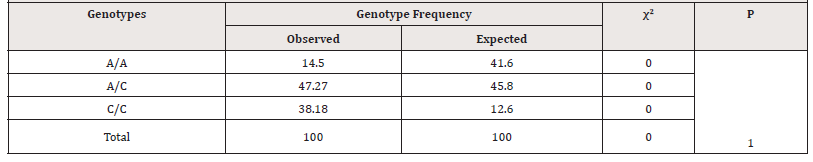
Table 3:Expected and observed frequency of distribution of genotypes by the HWE of the rs2018736 A456C polymorphism of the FBLN 5 gene in the main group of women with PHEGP
As follows from Table 3, the frequency of genotype distribution by the HWE of the rs2018736 A456C polymorphism of the FBLN 5 gene in the main group of patients, the observed frequency of A/A genotypes was found in 14.5%, heterozygous A/C genotypes was found in 47.03% and homozygous C/C genotype was found in 38.2%, respectively, while the expected frequency of genotypes of the A/A group was 41.6%, of the heterozygous A/C was found in 45.8%, and homozygous C/C was found in 12.6% of cases. Whereas in the control group, the observed and expected frequency of functional A/A genotypes of the FBLN 5 gene occurred in 64.8% and 58.3% of cases, respectively, and heterozygous variants of the A/C genotypes occurred in 29.7% and 36.14% cases, and homozygous unfavorable C/C variants were found in 5.4 and 5.6% of cases, respectively (Table 4).
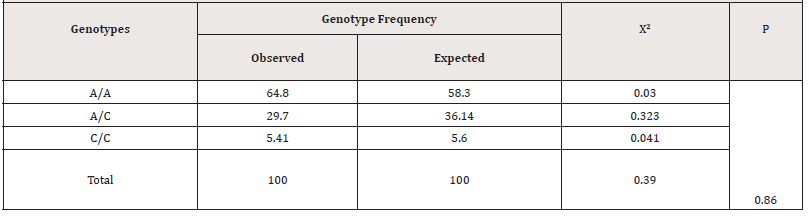
Table 4:Expected and observed frequency of genotype distribution by the HWE of the rs2018736 A456C polymorphism of the FBLN 5 gene in the control group.
A comparative analysis of the expected and observed genotype frequencies of this polymorphism revealed the absence of a statistically significant deviation of indicators (P>0.05) in all the studied groups, which indicates that the observed genotypes in the studied samples correspond to the Hardy-Weinberg equilibrium.
The study of the genetic structure of this marker revealed a relatively high level of expected A/C heterozygosity in the control and active treatment groups of patients (36.14% and 45.8%, respectively). The results obtained indicate the possibility of high frequencies of expected heterozygotes in the control group, rather than calculated heterozygotes. Considering the correspondence of the observed proportion of genotypes of the rs2018736 A456C polymorphism of the FBLN 5 gene in the studied samples to the Hardy-Weinberg equilibrium, our study indicates a possible connection between the functionally unfavorable allele “C”, leading to the replacement of A with C at position 456 of the amino acid sequence, with the risk of developing PHEGP after childbirth. At that, the risk of developing PHEGP in the presence of the variant allele of C polymorphism in the genome increases the risk by 7 times (OR=7.22). Thus, the C allele and hetero/homozygous genotypes of the rs2018736 A456C polymorphism of the FBLN 5 gene are significant markers of an increased risk of developing PHEGP in women of the Uzbek ethnic population. (P<0.004). (χ2=27.30; p<0.0006; OR=10.81; 95% CI 2.35-49.68). The A allele and the functionally favorable A/A genotype are reliable protective markers for the development of pathology (χ2=30.84; p<0.001; OR=6.37; 95%CI 3.21 -12.63).
Conclusion
The results of molecular genetic testing showed that studying the frequency of distribution of alleles of the rs2018736 A456C polymorphism of the FBLN 5 gene in the sample of women from the main group and in the population sample (control) showed that the favorable (functional) allele A in the control group of women without PHEGP occurred in 79.7% of cases (59/74), and in the main group of women with PHEGP, allele A was found in 38.2% of cases (42/110), which was 2.08 times lower compared to the control group. (χ2=30.84; p<0.001; OR=0.16; 95%CI 0.08-0.31). While the non-functional (mutant) allele “C” was detected in 68 cases in the group of women with PHEGP, which amounted to 61.8% (68/110), while in the group of control subjects without PHEGP, the mutant allele C was determined in 15 cases (15/74), which amounted to 20.3%. (χ2=30.84; p<0.001; OR=6.37; 95%CI 3.21 -12.63). The heterozygous A/C genotype of the association of the rs2018736 A456C polymorphism of the FBLN 5 gene was found in the main group of patients with PHEGP in 26 patients, which accounted for 47.3% of cases. Whereas in patients of the control group, this genotype A / C was found in 11 women, which accounted for 29.7% of cases, respectively, which was 1.6 times lower compared to the main group. (P<0.05) (χ2=27.30; p<0.0006; OR=2.12; 95% CI 0.88- 5.12).
The unfavorable homozygous C/C variant of rs2018736 A456C of the FBLN 5 gene was found in 21 patients in the main group, which was 38.2% (21/55), and in the control group, this genotype was found in 2 out of 37 women, which was 5.4 % of cases (5/37), respectively. (χ2=27.30; p<0.0006; OR=10.81; 95%CI 2.35-49.68).
Given that in the main group of women with PHEGP, the detectability of the unfavorable homozygous C/C variant of the FBLN 5 gene is high and exceeds that of control subjects by 7.07 times, the data obtained may indicate that the carriage state of the homozygous genotype of the rs2018736 A456C polymorphism of the FBLN5 gene may be a factor of predisposition to the development of this pathology, increasing its risk by 7.1 times (OR=10.81). Thus, the C allele and hetero/homozygous genotypes of the rs2018736 A456C polymorphism of the FBLN 5 gene are significant markers of an increased risk of developing PHEGP in women of the Uzbek ethnic population. (P<0.004). (χ2=27.30; p<0.0006; OR=10.81; 95% CI 2.35-49.68). The A allele and the functionally favorable A/A genotype are reliable protective markers for the development of pathology (χ2=30.84; p<0.001; OR=6.37; 95%CI 3.21-12.63).
References
- Atoyan MR (2005) Genetic determinants of genital prolapse and urinary incontinence in women: dis cand honey. Sciences pp. 128.
- Baranov BC (2009) The genetic passport is the basis of individual and predictive medicine. SPb pp. 528.
- Bagaev VM Omission, (1976) prolapse of the uterus and vagina. VM Bagaev, AM Avdeev. Paramedic and midwife 4: 30-32.
- Baisova EI (1999) The choice of the method of surgical treatment of uterine prolapse: dis. cand. honey. Sciences 94 p.
- Buyanova SN (2000) Principles of choosing the method of surgical correction of genital prolapse and urinary incontinence Mother and child: mater. P-th Ros. Forum S pp. 191-192.
- Glebova NN, Trubin VB, Latypov AS, Trubin TB (1997) Omission and prolapse of the internal genitalia of a woman. Ufa S 93-95.
- Safka Brozkova D (2020) Charcot Marie Tooth demyelinating neuropathy associated with FBLN5 mutations. Eur J Neurol.
- Kamoeva SV, Savchenko TN, Abaeva Kh A, Demura TA, Ivanova AV (2013) The role of matrix proteins Fbln-5 and LOXL-1 in the pathogenesis of pelvic organ prolapse. Russian Bulletin of an obstetrician-gynecologist 13(3): 33-37.
- TYu, Smolnova SV, Saveliev LI, Titchenko VL, Grishin NI, et al. (2001) Is genital prolapse a consequence of traumatic childbirth or generalized connective tissue dysplasia? Obstetrics and gynecology (4): S 33-37.
- Tiphaine Ravenel Bonetti, Anne Erpelding, Laxmi Raj Pathak (2004) Listening to "felt needs": investigating genital prolapse in western Nepal. Reprod. Health Matters 12(23): 166-175.
- Kulavsky VA, Ziganshin AM (2009) Influence of adverse lifestyle factors on the development of pelvic floor muscle failure and pelvic organ prolapse. Russian Bulletin of an obstetrician-gynecologist 4: S36- 40.
- Mamaeva AV (2007) Clinical prediction and prevention of posthysterectomy genital prolapse. diss. Candidate of medical sciences pp. 174.
- Tarabanova OV (2005) Loop operations (TVT, TOT) for stress urinary incontinence in gynecological patients: dis cand honey Sciences pp. 107.
- M Beer, A Kuhn (2005) Surgical techniques for vault prolapse: a review of the literature. Eur. J. Obstet. Gynaec. reproduction. Biol. 119(2): 144-155.
- Salah M Mawajdeh, Raeda J Al Qutob, Abdul M Farag (2003) Prevalence and risk factors of genital prolapse. A multicenter study. Saudi. Med J 24(2):161-165.
- (2020) Fibulin-5 protects the chondrocyte extracellular matrix by inhibiting the Wnt/β-catenin signaling pathway and alleviates osteoarthritis. GaoJ. B. idr. Eur Rev Med Pharmacol Sci.
- Ingrid E Nygaard, Rebecca McCreery, Linda Brubaker, Annamarie Connolly, Geoff Cundiff, et.al. (2004) Abdominal Sacrocolpopexy: A Comprehensive Review. Obstet. Gynecol 104(4): 805-823.
- Kolesnikova EI (2006) Long-term results of prevention of posthysterectomy prolapse of the vaginal dome in patients with genital prolapse. diss. Candidate of medical sciences pp. 146.
- Romikh VV, Sivkov AV (2005) Modern methods of urodynamic diagnosis of urinary incontinence in women. Obstetrics and Gynecology (5): S 53-56.
- Scotti RJ, Flora R, Greston WM, Budnick L, Hutchinson Colas J (2000) Characterizing and reporting pelvic pelvic floor defects: the revised New York classification. Int. Urogynecol. J. Pelvic Fioor Dysfiinct 11(1): 48-60.
- (2002) Gynecology according to Emil Nowak. M.: Practice pp. 896.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.